Biologics
AcuPac Advanced®
BONE VOID FILLER ALLOGRAFT


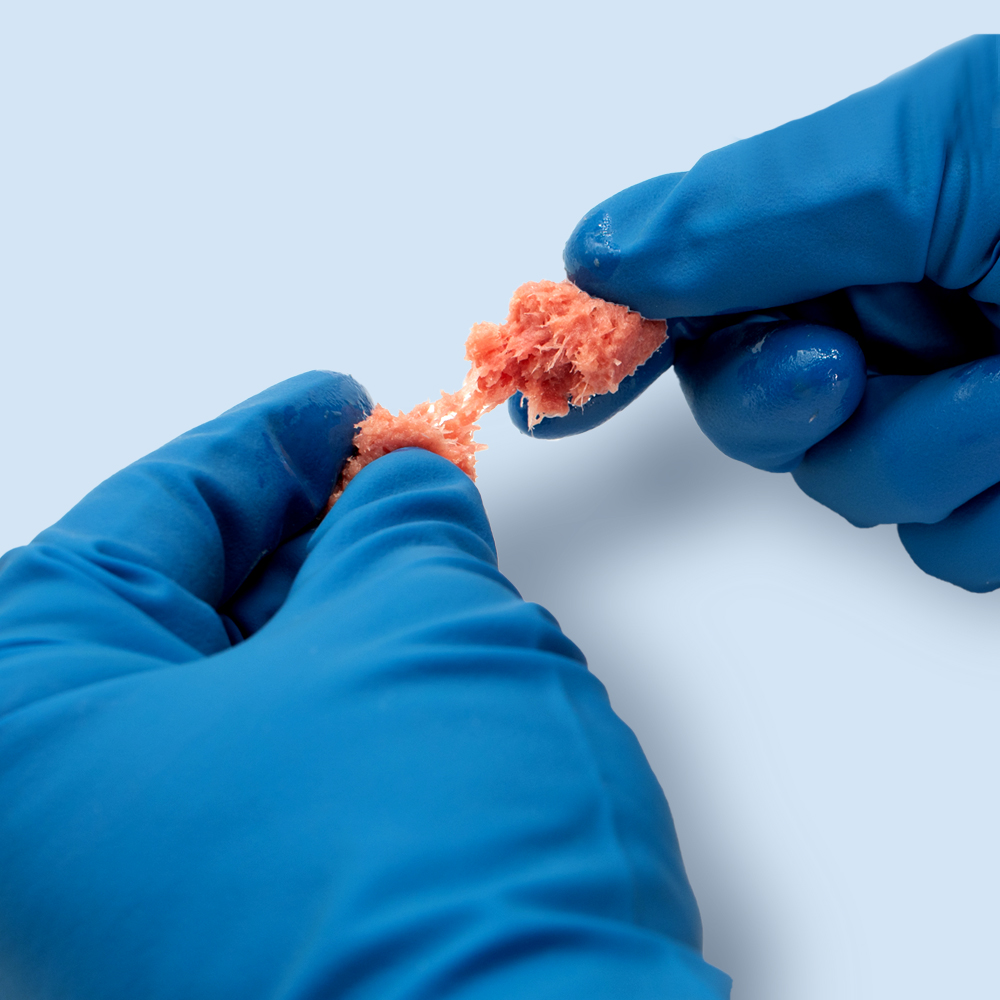
Description
AcuPac Advanced® is a premier human tissue allograft consisting of frozen cancellous bone and demineralized cortical fibers that function as a bone void filler to support bone repair.
Frozen & Ready-to-Use
AcuPac Advanced® lets you ensure the highest quality bone product for your patient. It goes through an exacting donor screening and testing process from an AATB-Accredited Tissue Bank.
AcuPac® Deformity
BONE VOID FILLER ALLOGRAFT FOR DEFORMITY APPLICATIONS

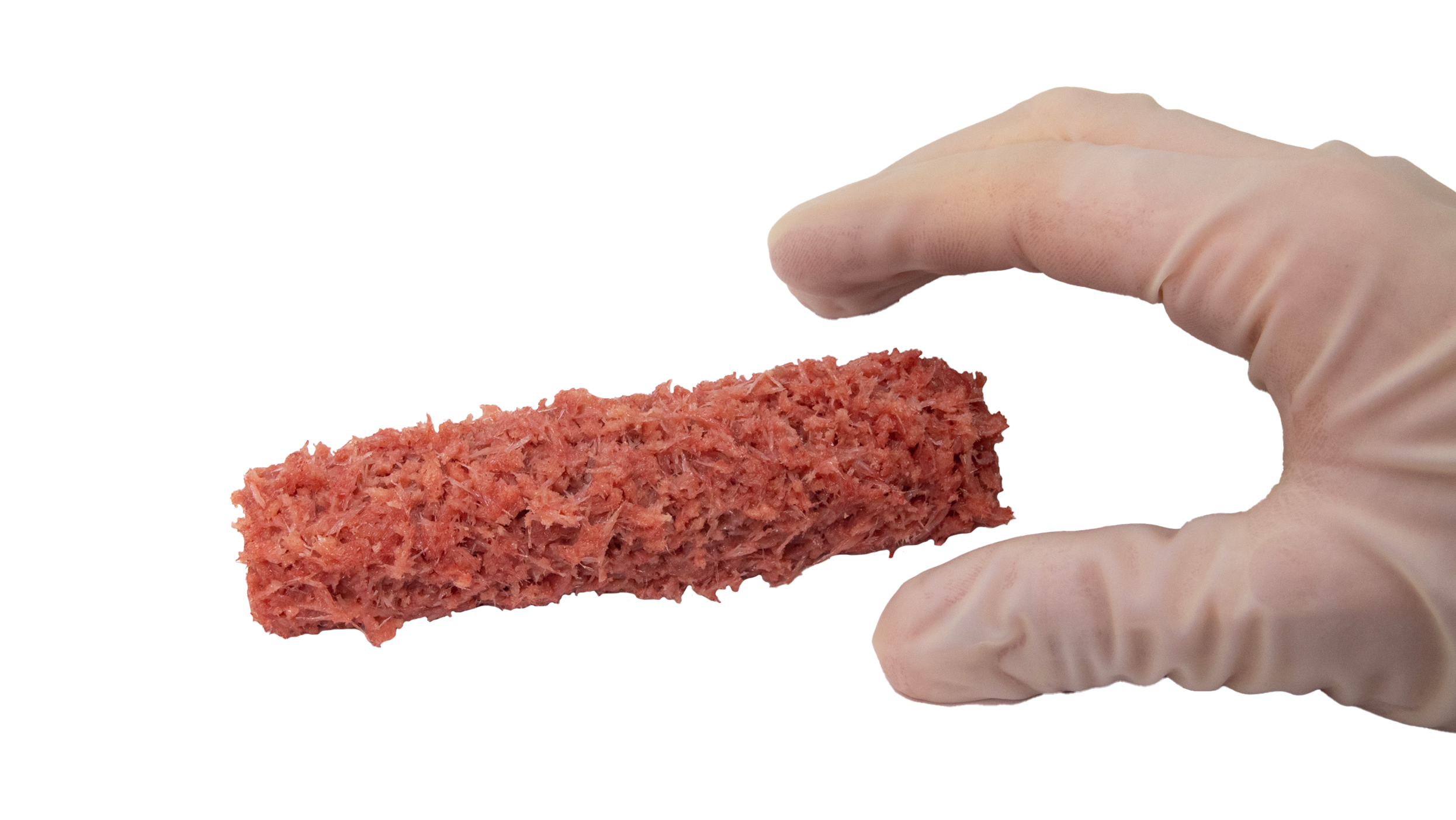
Description
AcuPac® Deformity is a refined alternative to autograft, with the additional benefit of demineralized cortical fibers. It is a natural bone void filler surgeons can use to promote rapid, complete bone regeneration.
Frozen & Ready-to-Use
AcuPac® Deformity provides maximum surgical flexibility. After thawing, the bone product has a fibrous cancellous autograft consistency that can fill in defects of all shapes and sizes.
AcuPure® DBM Putty
SUPPORTING BONE REPAIR WITH INNOVATIVE FLOWABLE, FORMABLE PUTTY.


Description
AcuPure® DBM Putty is a gamma-irradiated, sterile human tissue allograft consisting of 100% human demineralized bone allograft. These allografts function as a bone void filler to support bone repair.
Indications
AcuPure® DBM Putty is an allogeneic bone graft, which is used as bone void filler for bridging gaps in bone caused by surgical procedures, trauma, infection or excision of tumor(s). The allograft can be used in a number of orthopedic, spine, general and reconstructive surgical applications.
AcuPure® DBM Putty is intended for one-time use only, for a single patient, and by a licensed physician, dentist or podiatrist.
AcuPure® DBM Putty is supplied at ambient temperature and packaged sterile in a peel pouch contained within another peel pouch. Allograft volume is indicated on the package label.
AcuPure® Fiber
ALLOGRAFT BONE VOID FILLER WITH EASY TO MOLD FIBERS.



Description
AcuPure® Fiber is a gamma-irradiated, sterile human tissue allograft consisting of 100% human demineralized bone allograft. These allografts function as a bone void filler to support bone repair.
Indications
AcuPure® Fiber is intended for one-time use only, for a single patient, and by a licensed physician, dentist or podiatrist. AcuPure® Fiber is supplied at ambient temperature and packaged sterile in a peel pouch contained within another peel pouch. Allograft volume is indicated on the package label.
AcuPatch®
AMNION TISSUE PATCH
Description
AcuPatch® Amnion Tissue is a protective aminion patch. Amniotic tissues are allografts, regulated by the FDA as a human cell, tissue, and cellular and tissue based product (HCT/P) under 21 CFR Part 1271 and Section 361 of the Public Health Service Act.
Indications
AcuPatch® Amnion Tissue is desiccated to allow for storage at room temperature and immediate use off the shelf (no preparation required). The reduced moisture in the graft makes the tissue easy to handle and hydrates when placed in the wound environment to readily adhere to the wound site.
